Innovations in Endovascular Aneurysm Repair
April 16, 2018 | Lindsay Morgan
Every year, 200,000 people in the United States are diagnosed with an abdominal aortic aneurysm (AAA)—a bulging dilation or ballooning in the wall of a blood vessel, usually an artery, that is due to weakness or degeneration that develops in a portion of the artery wall. A ruptured AAA, which can cause fatal bleeding, is the 15th leading cause of death in the country, and the 10th leading cause of death in men older than 55 ¹ .
Historically, AAAs were repaired via open surgery (open aneurysm repair, OAR), in which surgeons make a large incision in the abdomen and manually sew a reinforcement graft into place. In addition to long hospital stays and recovery times, the operation carries an approximately 5% risk of mortality and a morbidity rate of between 30-40%.
Beginning in the early 1990s, a minimally invasive procedure called Endovascular Aneurysm Repair (EVAR) has been increasingly used.

In the EVAR procedure, a stent graft (a fabric tube supported by metal wire stents that reinforces the weak spot in the aorta) is inserted into the aneurysm through small incisions in the groin. Endovascular repair is associated with extremely low perioperative mortality and has a substantially shorter recovery than the conventional open surgical approach.
John Lane, Professor of Surgery and Interim Chief of the Division of Vascular and Endovascular Surgery, says: “With EVAR, the surgery takes a couple of hours, the patient goes home the next day. We do it percutaneously, so instead of having any major incision on the body, you have two punctures.”
EVAR has been enthusiastically adopted: by 2004, EVAR exceeded open repair for the first time.
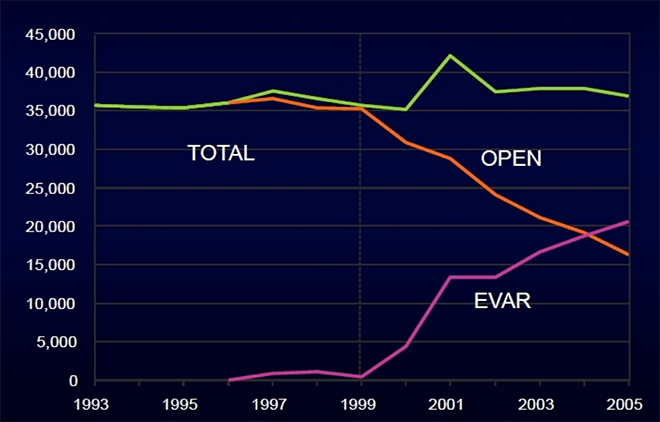
But evidence shows that EVAR is not as durable as open repair. At least 1 in 8 EVAR patients will undergo secondary intervention within 5 years, and two notable randomized control trials (RCTs)—the EVAR1 trial and the DREAM trial—have shown that EVAR is worse at the one thing it ought to be successful at: aneurysm-related mortality (ARM).
“EVAR has an early survival benefit but an inferior late survival compared with open repair, which needs to be addressed by lifelong surveillance of EVAR and prompt re-intervention if necessary” ( Lancet ).
The reasons for this are not completely clear, but evidence suggests two factors driving poor results:
- Durability issues with old EVAR devices
- Weak sac management: OAR ligates (i.e., closes off) branch vessels whereas EVAR does not
Says Lane: “With EVAR that there are device failures, especially with the earlier devices. We’re seeing leaks, aneurysm expansions, late ruptures, late complications. The technology has been really revolutionary, so a lot of the research now is focused on: how can we make it better?”
From EVAR to EVAS: Nellix, a Polymer-Based Approach to Sealing
Researchers at UCSD are evaluating ways to address these problems, including through aneurysm sac embolization and development of novel device called Nellix.
The Nellix System for endovascular aneurysm sealing (EVAS) is a novel approach to AAA treatment whereby polymer is used to fill the AAA sac. The polymer is injected ex vivo and molds to the patient’s anatomy inside the body. In this way, it accommodates for irregular anatomy, and can close off branch vessels feeding into the aneurysm sac (one of the causes of failures). Furthermore, unlike a stent graft, which holds itself in place by pushing outward with a spring-loaded force, when the polymer sets, it ceases to expand. Thus, it depressurizes the aneurysm sac.
The EVAS2 trial, a confirmatory trial to test Nellix, is kicking off this spring Data from the initial Nellix trial showed that the device outperformed every other device in every respect: it had a lowest endo-leak rate; it was the simplest device to put in place; it had the lowest procedure times, the least amount of radiation exposure, the least amount of contrast delivered.
“Everything was extremely rosy,” says Lane. “This looked like the simplest and most effective device that was out there.”
However, the FDA did not approve the device. Instead, they required another year of data. The decision proved prescient.
“The two-year data showed device failures that we frankly didn’t expected to see.” In certain anatomies—about 70% of the study sample—there was an increase in aneurysm size, along with migration of devices.
The company who owns the technology, Irvine-based Endologix, Inc., decided to change the Indications for Use (IFU) or anatomic criteria by which the graft is approved—essentially asking the FDA to approve the device based on a subset of data. The FDA pushed back, requiring that the trial be re-run in the smaller subset of patients with whom the device had worked well. The EVAS2 IDE Multicenter Safety and Effectiveness Confirmatory Study ("EVAS2"), thus, will prospectively evaluate the refined IFU and the Nellix Gen2 EVAS System. The study is approved to enroll up to 90 primary patients, with one-year follow-up data required for the Pre-market Approval Application (PMA).
“You want a device that will work in almost everyone, so the results raised concern. I think the technology will, ultimately, be transformative, but is the current graft design going to be the end all be all that we thought it was going to be after one year? The answer to that is no. But we can take what we’ve learned and create a second-generation device. It’s interesting. We’re learning.”
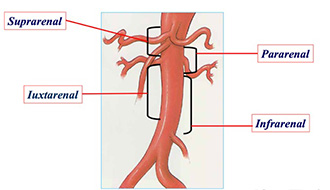
One of the potentially powerful uses of the Nellix device is being tested in another clinical trial, which is just getting off the ground, to test use of the device in complex cases. As aneurysms progress and move up the body, they begin to involve major branches—to the kidney, intestines, spleen and liver. These juxtarenal aneurysms require highly advanced surgical skills to tackle and there is only one device on the market aimed at fixing them. That device is based on the stent graft design: it consists of a tube pocked with holes, or fenestrations, through which stents must be placed and sealed. These stents protrude from the main stent like a chimney and are thus known as “chimney” EVAS or chEVAS. It’s a highly complicated procedure—so much so that only four of the procedures have been done in the United States; Dr. Lane has done two of them.
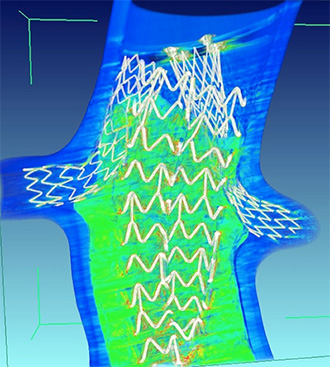
The Nellix polymer technology may simplify this. Rather than having a stent that has a hole in it and putting a wire through a hole—a difficult task—surgeons can place stents into the vessels, and then advance the polymer devices around those stents. The polymer will mold around the stents and do the sealing automatically.
If the FDA approves Nellix based on the EVAS2 trial, the technology will be available for use in complex cases as well.
Flow Modeling: Moving Towards A Personalized Aneurysm Sac Management Protocol
UC San Diego researchers are also moving ahead with projects aimed at modeling the stresses on the wall of an aneurysm, in an effort to create individual risk profiles, in an incredible example of personalized medicine. Working with Dr. Juan Lasheras, Chair, School of Engineering, Dr. Andrew Barleben and collaborators will evaluate failure modes due to lack of sac management, embolization strategies, and create virtual flow models.
Says Barleben: “Once the flow model is created we could apply the same parameters on a case by case basis (aneurysm by aneurysm basis). Then we could create a personalized aneurysm sac management protocol that would help us predict when and where to embolize.” The project is being funded by the Altman Clinical and Translational Research Institute (ACTRI) [link].
A Place Ripe For Scientific Exploration
Vascular surgery is replete with scientific-technological inquiry, and UC San Diego’s Division of Vascular and Endovascular Surgery have active major clinical trial in every area of vascular disease—from treating carotid disease and aneurysms, to treating lower extremity disease, and even dialysis and treating high blood pressure.
Says Lane: “We have a very busy system here. Our clinical volume is high, so we’re the number one enroller in the trial [EVAS2]. We have more aneurysm patients cycling through the VA than any other trial site in the country. We also have good clinical research machinery here. The VA has a very organized and well-oiled machine for clinical research trials, and that’s one of the reasons why we are so diversified in trials. You move to Napa Valley, you grow grapes. Research is what you do here.”
Learn more about clinical trials in the Department of Surgery
