Guiding Lights: UC San Diego Launches Center For Fluorescence-Guided Surgery
December 5, 2019
Story by Jackie Carr
In the 1990’s, Nobel Laureate Roger Tsien, PhD, took some of the first dramatic steps to revolutionize the field of surgery by introducing the concept of using fluorescence to light up cells for easy identification. What had started with bioluminescent jellyfish had moved through labs and Petri dishes to become a lifesaving imaging tool for surgeons — now in everyday practice at UC San Diego Heath.
 “The new Center for Fluorescence-Guided Surgery at UC San Diego Health builds upon Dr. Tsien’s work here and his legacy,” said Santiago Horgan, MD , the center’s director and chief of minimally invasive surgery. “This is the first dedicated center in the country to deliver a new caliber of surgical accuracy, allowing UC San Diego Health doctors to treat patients with tumors and complex diseases of all types with GPS-like precision, easily identifying cancerous or critical tissues throughout the body because they literally glow.”
“The new Center for Fluorescence-Guided Surgery at UC San Diego Health builds upon Dr. Tsien’s work here and his legacy,” said Santiago Horgan, MD , the center’s director and chief of minimally invasive surgery. “This is the first dedicated center in the country to deliver a new caliber of surgical accuracy, allowing UC San Diego Health doctors to treat patients with tumors and complex diseases of all types with GPS-like precision, easily identifying cancerous or critical tissues throughout the body because they literally glow.”
Fluorescence-Guided Surgery, or FGS, can be used in open, laparoscopic and robotic surgeries to identify critical structures, tumor margins and blood flow in tissues, in real-time. FGS is typically performed by injecting contrast agents or targeted imaging probes into the blood stream of patients just before surgery. Using specialized cameras and light sources that can detect light in multiple wavelengths, surgeons can visualize tumor anatomy or other critical structures for more precise surgery.
 The new Center for Fluorescence-Guided Surgery builds upon Roger Tsien’s groundbreaking work at UC San Diego.
The new Center for Fluorescence-Guided Surgery builds upon Roger Tsien’s groundbreaking work at UC San Diego.
“Intraoperative fluorescence imaging offers the benefits of low cost, ease of use, no reliance upon damaging ionizing radiation and enhanced safety through visualization,” said Michael Bouvet, MD , center co-director and former president of the International Society of Fluorescence Guided Surgery. “Improved visualization of the tissues can reduce damage to important normal structures, such as nerves, blood vessels, ureters and bile ducts.”
“UC San Diego Health uses fluorescence imaging to optimize a full spectrum of surgeries from common procedures, such as gallbladder removal and weight loss surgery to the most complex neurosurgical procedures,” added Horgan, also director of the Center for the Future of Surgery at UC San Diego School of Medicine. “This approach is fundamentally changing how surgery can be performed more safely and accurately for patients.”
The most commonly used fluorescent marker is Indocyanine Green (ICG). Developed in the 1950s, ICG is a water-soluble fluorescent molecule with a proven safety profile that binds to plasma proteins. ICG is used as a marker in the assessment of perfusion (passage of blood or other fluids) in tissues and organs in many areas of medicine. The light required to trigger fluorescence is generated by a near-infrared radiation source attached to a camera, which tracks and documents fluorescence absorption in real time.
Bouvet and fellow surgeon Quyen Nguyen, MD, PhD , were early innovators of FGS. In 2008, Bouvet, Robert M. Hoffman, PhD, and colleagues first described the use of fluorophore-conjugated antibodies for surgical navigation of colon and pancreatic tumors in mouse models. They subsequently adapted the technology for minimally invasive laparoscopic surgery. Bouvet now routinely uses fluorescence-guided surgical techniques in parathyroid, adrenal and esophageal surgeries.
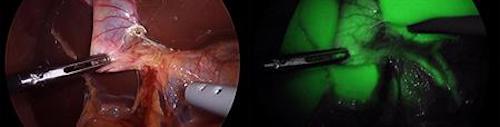 UC San Diego Health surgeons use fluorescence to identify key structures within organs such as the gallbladder.
UC San Diego Health surgeons use fluorescence to identify key structures within organs such as the gallbladder.
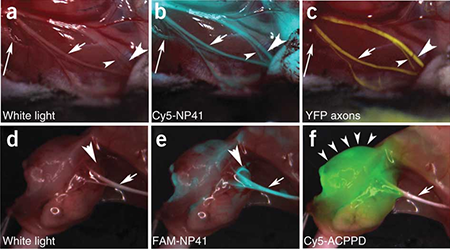
In 2004, Nguyen and Tsien teamed up to develop one of the first targeted injectable fluorescent molecules to specifically highlight tumors and hard-to-see peripheral nerves.
“The remarkable thing about this fluorescence is not just that it’s bright, but that it can go through tissue, so you can see a tumor or nerves even if they are buried deep in other tissues,” said Nguyen, head and neck surgeon. “Fluorescently labeled tissue just glows, right there in the operating field, enabling surgeons to take out every last bit of tumor or avoid inadvertent injury to critical structure such as nerves.”
UC San Diego completed the first-in-human Phase I clinical trial of Tsien and Nguyen’s fluorescent peptides targeted for tumor margins in 2017. The study allowed breast tumors to be visualized in the operating room. Anne Wallace, MD , principal investigator, was able to achieve separation of tumor from adjacent tissues using a fluorescent image in both primary tumors and in lymph nodes in patients undergoing surgical resection of breast cancer.
“The use of fluorescence is an absolute game-changer for cancer surgeries,” said Wallace, director of the Comprehensive Breast Health Center at UC San Diego Health. “The evolution of Dr. Tsien’s work in the lab to our operating rooms at UC San Diego Health is a combination of translational and personalized medicine at its best.”
As fluorescence imaging evolves and advances, researchers say more targeted molecules will be developed and designed to individual patients’ disease profiles.
“While many operations at UC San Diego Health use fluorescence guidance now, imaging techniques and molecule development continue to progress through the research of the Center for the Future of Surgery at the T. Denny Sanford Medical Education and Telemedicine Center,” said Horgan.
 Sonia Ramamoorthy, MD , chief of colorectal surgery at UC San Diego Health, agreed: “This technology will change the way we do surgery now and in the future. Fluorescent guidance has the potential to improve patient outcomes by making identification of cancer resection margins easier to see. Additionally, through enhanced visualization of critical anatomy with fluorescence, we can reduce morbidity from surgical intervention.”
Sonia Ramamoorthy, MD , chief of colorectal surgery at UC San Diego Health, agreed: “This technology will change the way we do surgery now and in the future. Fluorescent guidance has the potential to improve patient outcomes by making identification of cancer resection margins easier to see. Additionally, through enhanced visualization of critical anatomy with fluorescence, we can reduce morbidity from surgical intervention.”
Ramamoorthy is principal investigator of an on-going Phase III Clinical Trial using SGM-101, a novel fluorescent tumor-specific antibody, to improve surgical outcomes in colorectal cancer patients.
Members of the new center include: Sarah Blair, MD; Bouvet; Ryan Broderick, MD; Jill Buckley, MD; Bryan Clary, MD; Amanda Gosman, MD; Horgan; Christopher Kane, MD; Alexander Khalessi, MD; Frederic Kolb, MD; Michael McHale, MD; Kristin Mekeel, MD; Nguyen: Ryan Orosco, MD; Ramamoorthy; Chris Reid, MD; Cheryl Saenz, MD and Wallace.
