FDA approves tumor localization device based on UC San Diego microparticle technology
March 1, 2019 | By Lindsay Morgan
When someone has a tumor, the job of the surgeon is to identify it in the body, and remove all of it—every last microscopic trace—both to ensure that all the cancer is gone and to reduce the risk of recurrence.
Cancer screening is better than ever at identifying tumors, including very small ones. But small, nonpalpable tumors remain challenging for surgeons to identify (and thus eliminate completely, microscopically) in the operating room.
 Dr. Sarah Blair, Professor of Surgery in the Division of Breast Surgery, who specializes in breast cancer, melanoma, sarcoma and soft tissue tumors, has always had an interest in surgical margins and how to ensure patients don't need second operations. For breast cancer patients in particular, a second or third operation can result in mastectomy—a heartbreaking trauma for patients.
Dr. Sarah Blair, Professor of Surgery in the Division of Breast Surgery, who specializes in breast cancer, melanoma, sarcoma and soft tissue tumors, has always had an interest in surgical margins and how to ensure patients don't need second operations. For breast cancer patients in particular, a second or third operation can result in mastectomy—a heartbreaking trauma for patients.
"I had a recent case, a young girl who was 34," says Blair. "She had a relatively large tumor, and when they did the MRI, they found additional abnormal cells, which they biopsied. During the biopsy, they placed four 5-millimeter clips to improve localization at the time of surgery. Then she got chemotherapy, which shrunk the tumors, so that by the time we operated, all that was left to guide us to the potentially remaining cancerous cells were those clips. One of them was very difficult to localize during surgery, which resulted in microscopic traces of the tumor being left behind. Now she faces another surgery and a possible mastectomy."
The existing technology for localizing breast tumors includes use of clips with b-mode ultrasound response, wires, and dyes. Clips with b-mode ultrasound can be difficult to see, and wires run the risk of migration, are uncomfortable for patients, and create logistical issues with coordinating two procedures on the same day, typically in different physical locations (breast imaging in an outpatient location and surgery in the hospital or surgery center). For lung cancer, wires in the lung cavity are dangerous—if a wire moves, it can hit a blood vessel or puncture the lung.
Dr. Blair had tried to overcome these challenges in breast surgery by creating an automated system to check margins during surgery but it was complicated and cumbersome.
"So I thought, what if we could just localize the tumor better?"
That's when Dr. Blair began looking into a nano tumor center funded by an institutional grant that UC San Diego had received from the National Institute of Health. This grant supported chemists, engineers and clinicians innovating the use of nanotechnology to fight cancer. A group of chemists were making gas-filled silica particles intended to be used as an ultrasound contrast agent, "but they were too stationary," says Blair." "They were supposed to flow through the bloodstream and get to the tumor but they didn't move."
What didn't work for their purposes ended up working for Blair's. "I thought it might be a great technology for localizing tumors — because in our case, we want the particles to be stationary. And that's how this project got started."
In 2011, Dr. Blair was awarded an NIH grant to continue research into the use of the stationary particles for tumor localization. Then in 2016, View Point Medical, a translational medical device company, licensed the microparticle technology to use as a key component of their SignalMark tissue marker line: a new, low-cost, tissue marker system designed to enable lesion localization during biopsy site marking. Dr. Blair's microparticles enable SignalMark's distinct doppler ultrasound response, setting the line apart from other known biopsy and localization tissue markers.
To evaluate the intraoperative localization value of the SignalMark tissue markers in multiple organs, Dr. Blair and her team conducted a study in both breast and lung. [1] The markers were deployed in twelve breasts of lactating pigs and subsequently identified using a standard ultrasound machine on color doppler mode by three surgeons blinded to marker location. The surgeons were timed in performing this task. The surgeons also were timed in locating HydroMark breast markers, a commercially available marker with b-mode ultrasound functionality. To demonstrate efficacy in lung parenchyma, a second cohort of pigs underwent lung injections. There are currently few effective methods for localizing thoracic tumors, but increased availability of CT scan screening programs means that surgeons are increasingly finding smaller lung tumors, underscoring the need for localization devices.
The results of the experiments were promising. In breast tissue, the SignalMark markers were more rapidly and accurately identified than the commercially available HydroMARK clips. In the lung, all 10 SignalMark markers were visible both on Doppler ultrasound and on the surface of the lung at the time of placement, and at the 7- and 21-day time points.
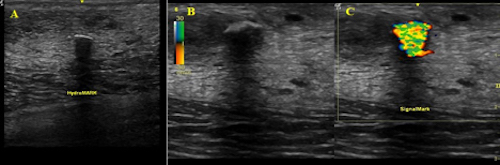
FIG. 1 Images of (a) HydroMARK and (b) SignalMark obtained with B-mode ultrasound. (c) SignalMark’s appearance using Doppler ultrasound
Says Blair: "With SignalMark, we get such a bright ultrasound signal; it's so much better than trying to localize tumors with the alternatives, like painful wires. Being able to place the marker weeks before surgery will reduce scheduling conflicts in the breast center and simplify the clinical workflow."
The results suggest that the technology has the potential to make it easier for surgeons to locate non-palpable breast and lung tumors using ultrasound guidance in the operating room during an actual procedure. (See Doppler Ultrasound-Visible SignalMark Microspheres are Better Identified than HydroMARK® Clips in a Simulated Intraoperative Setting in Breast and Lung Tissue .)
The SignalMark technology received FDA approval for use in the lung in December 2018. View Point Medical is in the midst of Series B financing, and in process for expanded clinical indications with FDA. The lung marker will begin human use in early 2019.
"Distinct, reliable, and simple localization is essential to successful tumor resection," says Mayah Klain, Director of Marketing at View Point Medical. "With SignalMark, we have an opportunity to decrease the delay in early stage cancer treatment, improving outcomes for patients while lowering overall healthcare costs for providers."
For Dr. Blair, seeing the project through to this stage is gratifying: "This is something I've been working on for a long time and it's really exciting to translate the technology into a tool that will improve patient outcomes."
[1] This study was funded by Viewpoint Medical. Dr. Blair has a family member with an equity interest in Nanocyte Medical, Inc., a company that may potentially benefit from the research results. H.O.F. is a consultant. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict of interest policies.
